Palladium
Our Brochures
Contact Us
Social Media
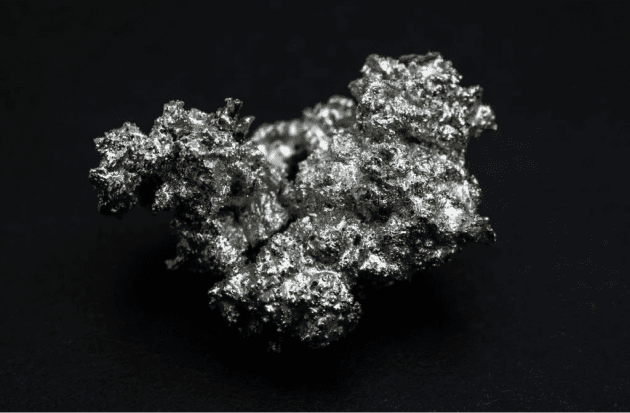

Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into nontoxic substances (nitrogen, carbon dioxide and water vapor).
Palladium Characteristics
Palladium is a group 10 element with a unique 5s0 electron configuration, making it the heaviest element with only one incomplete electron shell. It’s a soft, silver-white metal resembling platinum but is the least dense and has the lowest melting point in the platinum group. It is soft and ductile when annealed but gains strength when cold-worked. Palladium is stable in air and does not tarnish at standard temperatures; it dissolves in concentrated acids and aqua regia. It can exhibit superconductivity when subjected to alpha particle bombardment at low temperatures.
Unique 5s0 configuration
Silver-white metal
Least dense
Acid-soluble
Superconductivity
Carbon ComponentsComposition of coalScarcity is one of the most influential factors determining a metal’s value. This is a positive for palladium which is 30 times rarer than gold.
Plus, restrictive environmental policies limiting the extraction and mining of the metal have further constricted global supplies. Palladium’s relative rarity makes it a valuable investment option when used to strategically diversify a Precious Metals portfolio.
Most palladium is used in catalytic converters for cars. It’s also found in jewelry, dental fillings, and white gold alloys. In electronics, it’s used in ceramic capacitors in laptops and mobile phones. Palladium serves as an effective catalyst for hydrogenation and dehydrogenation processes.
Element Properties
Atomic number
Atomic weight
Melting point
Boiling point
Specific gravity
Oxidation states
Electron configuration
78
195.09
1,769 °C (3,216 °F)
3,827 °C (6,920 °F)
21.45 (20 °C)
+2, +4
[Xe]4f145d96s1
